
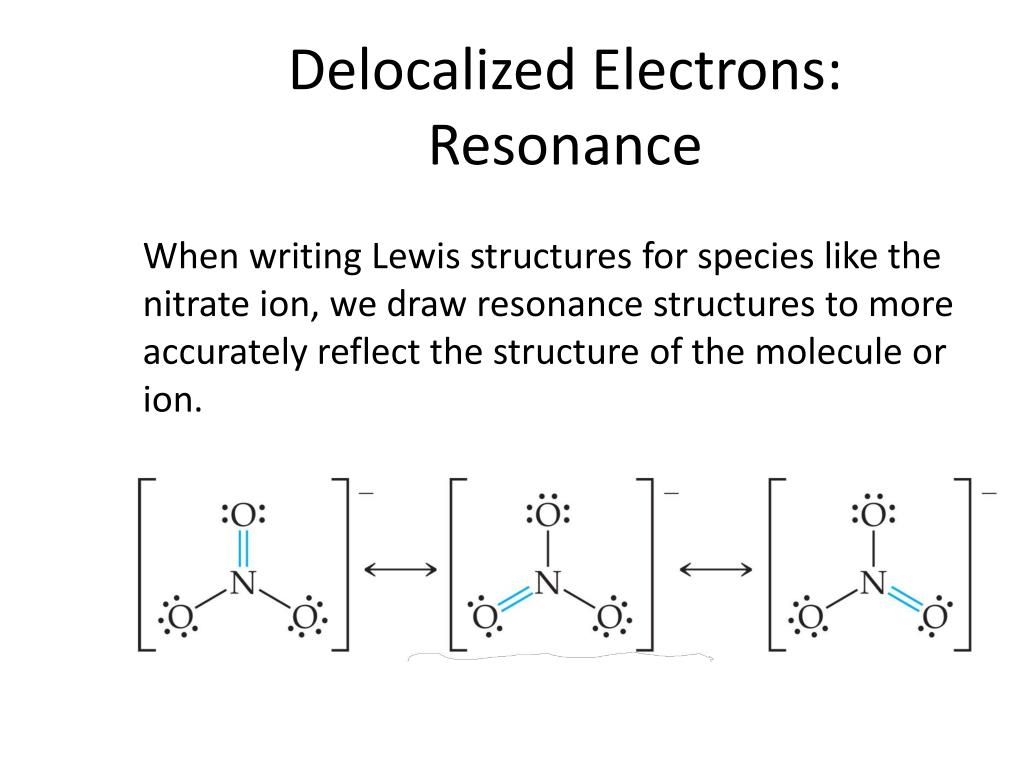
Provide 3 other examples of azo dyes (Chemdoodle drawn formulas. Benzene and cyclohexane have a similar structure, only the ring of delocalized electrons and the loss of one hydrogen per carbon distinguishes it from cyclohexane. We assume that σ-electrons are localized and π-electrons are delocalized in the ring.Įach carbon atom promotes one electron from its $\mathrm$ interaction.) The orbital on the right is exactly opposite (orthogonal) to that. CHM2211L2As a consequence of -delocalization, aryl azo compounds have rich colors. The difference is that the spaces are distributed differently: there are >6 spaces on the benzene ring and <2 spaces on the oxygen atom for them. In phenol, there are eight -electrons in total and eight spaces for them. Resonance contributors are imaginary, but the. Resonance Contributors and the Resonance Hybrid.
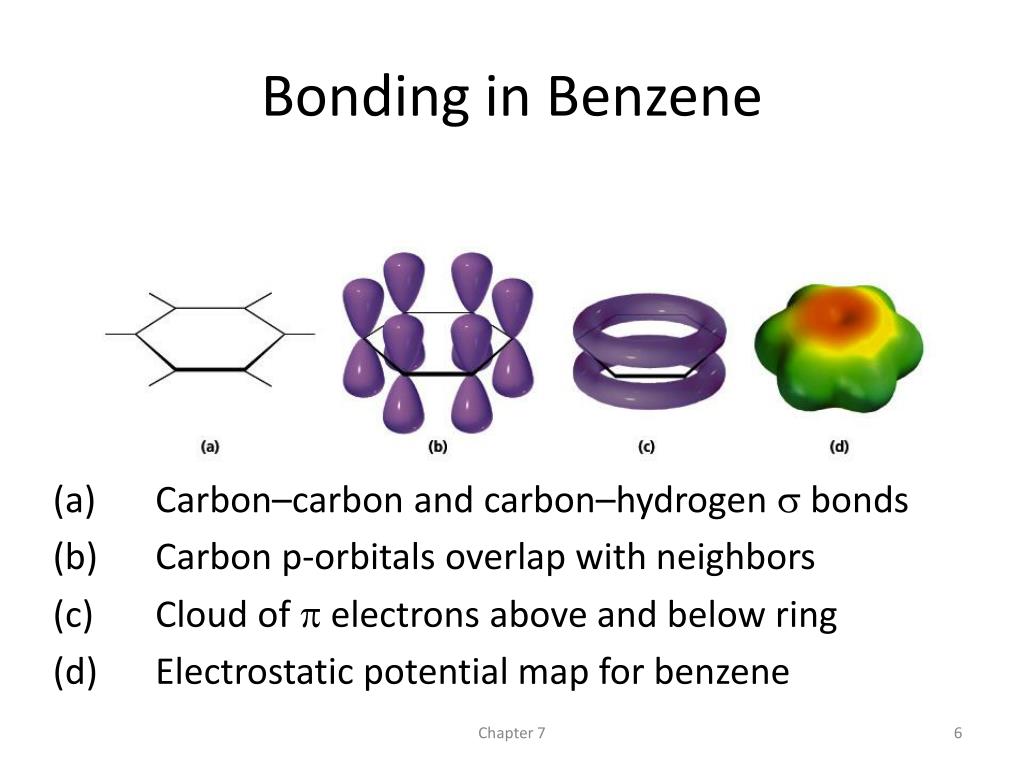
Benzene Each electron is shared by all six carbons The electrons are delocalized. Methane Benzene Water dimer Optimize Atoms. In the 30’s, x-ray diffraction showed: Benzene is a planar, 6-membered ring All C-C bonds are equal in length, in between the length of C-C and CC. Each carbon atom promotes one electron from its s orbital to the empty 2 p orbital. We assume that -electrons are localized and -electrons are delocalized in the ring.

Benzene, due to symmetry of its resonating structures, is simple enough.
Chemdoodle delocalized benzene free#
Benzene, due to symmetry of its resonating structures, is simple enough. In benzene itself, there are no substituents and thus there's no problem having six -electrons into six spaces. There is only a limited number of free calls to the ChemDoodle algorithm. Benzene and nitrate ion are given in my textbook as examples for the delocalization of -electrons. sy2), Tripos Sybyl Line Notation (.sln), Beilstein ROSDAL (.ros), XYZ Files (.Benzene and nitrate ion are given in my textbook as examples for the delocalization of π-electrons. mmod), Schrödinger Maestro (.mae), Standard Molecular Data (.smd), Tripos Mol2 (.mol2. Affordable and used by thousands of scientists around the world. Though it is highly stable, it undergoes addition and oxidation reaction under specific conditions. Benzene ring is stabilized by delocalized electrons. So electrophilic substitution reaction occurs in benzene. Therefore, when electrons are close together they cause repulsion and make the molecule kinetically unstable. Benzene contains delocalized -electrons which make the ring to act as an electro rich centre. Even in penta-1,4-diene, the electrons are still localized. Answer (1 of 2): You must be knowing that like charges repel. > In a molecule like ethylene, the electrons in the bond are constrained to the region between the two carbon atoms.
Chemdoodle delocalized benzene software#
ent), RCSB Protein Data Bank Markup Language (.xml. Chemical drawing and publishing software for desktop, web and mobile. A delocalized bond is a bond in which the electrons are free to move over more than two nuclei. mmcif), RCSB MacroMolecular Transmission Format (.mmtf), RCSB Protein Data Bank Files (.pdb. rd), MDL RXNFiles, both V2000 and V3000 connection tables (.rxn), MMI SketchEl Molecule (.el), Molinspiration JME String (.jme), RCSB Binar圜IF (.bcif), RCSB Macromolecular Crystallographic Information File (.cif. They can’t be in contact with each other. dx), ISIS Sketch File (.skc), ISIS Sketch Transportable Graphics File (.tgf), MDL MOLFiles, both V2000 and V3000 connection tables (.mol. Is CO2 delocalized The p-orbitals of the carbon atom are not delocalized by CO2. smiles), IUPAC InChI (.inchi), IUPAC JCAMP-DX (.jdx. Read and write many popular chemical file types for working with the applications you use:ĪCD/ChemSketch Documents (.sk2), ChemDoodle Documents (.icl), ChemDoodle 3D Scenes (.ic3), ChemDoodle Javascript Data (.cwc.js), CambridgeSoft ChemDraw Exchange (.cdx), CambridgeSoft ChemDraw XML (.cdxml), Crystallographic Information Format (.cif), CHARMM CARD File (.crd), ChemAxon Marvin Document (.mrv), Chemical Markup Language (.cml), Daylight SMILES (.smi. For instance, in the sketcher above, the query structure defines any molecule with a benzene ring where a halogen (x) is ortho to a saturated oxygen, sulfur or.


 0 kommentar(er)
0 kommentar(er)
